REFERENCES for introduction text
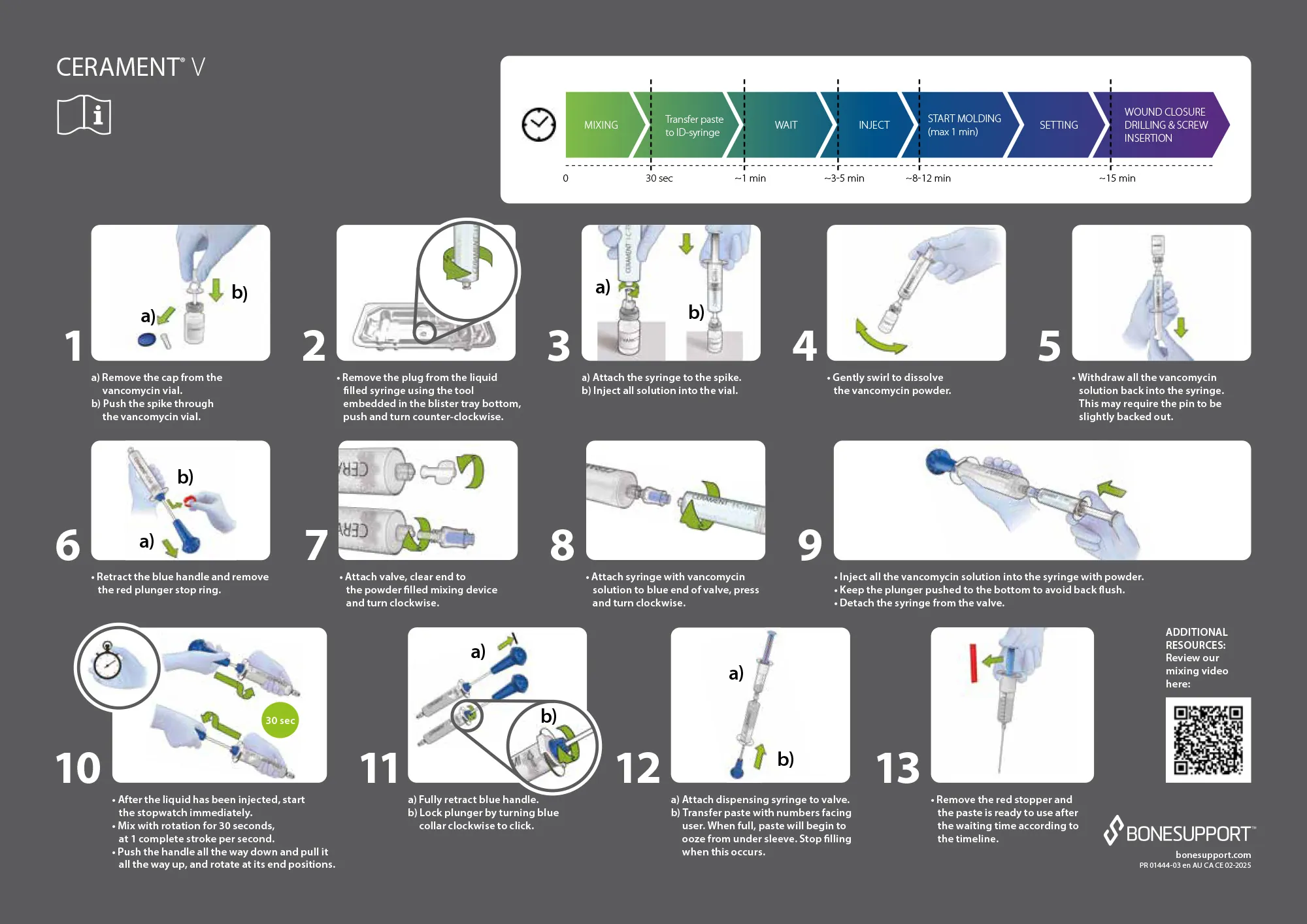
CERAMENT® V Instructions for Use.
REFERENCES for remodels into host bone within 6-12 months
Ferguson et al. ‘Radiographic and histological analysis of a synthetic bone graft substitute eluting gentamicin in the treatment of chronic osteomyelitis.’ J. Bone Joint Infect. 2019;4(2):76-84.
McNally et al. ‘Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite – A prospective series of 100 cases’. Bone Joint J 2016;98-B:1289–96.
Hettwer et al. ‘Establishment and effects of allograft and synthetic bone graft substitute treatment of a critical size metaphyseal bone defect model in the sheep femur’. APMIS 2019; 127:53–63.
REFERENCES for injectable and flowable
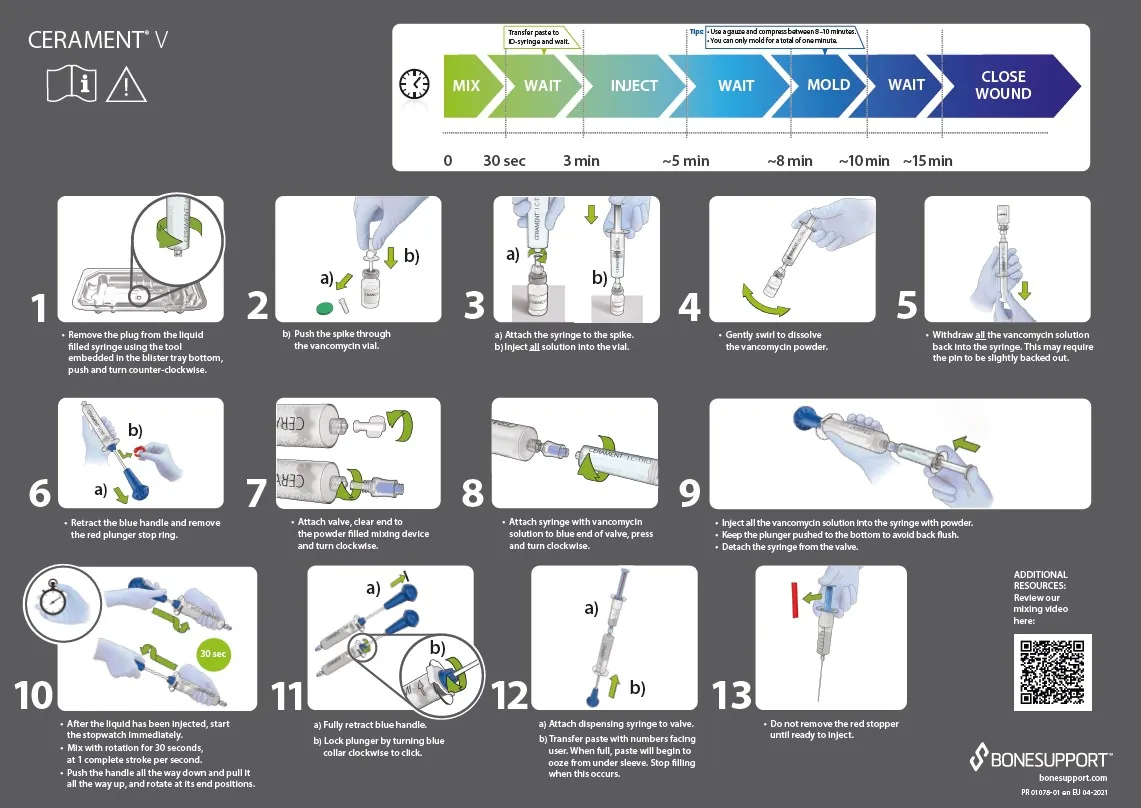
CERAMENT® V Instructions for Use.
Nilsson, M. ‘Injectable calcium sulphate and calcium phosphate bone substitutes’. Lund University. 2003. ISBN: 91-628-5603-0.
Karlsson et al. ‘Mechanical characteristics of a ceramic bone substitutes loaded with vancomycin’. 22nd European Conference on Biomaterials (ECB), Lausanne, Switzerland, 7-11th September 2019.
REFERENCES for vancomycin elution above MIC that is reliable and consistent
Stravinskas et al. ‘Vancomycin elution from a biphasic ceramic bone substitute.’ Bone Joint Res 2019;8:49–54.
Colding-Rasmussen et al. ‘In-vivo and in-vitro evaluation of vancomycin and gentamicin elution from bone graft substitutes’, European Bone and Joint Infection Society (EBJIS) 35th Annual Meeting, Oxford, UK, September 2016.
Lindberg. ‘In vitro characterization of a vancomycin eluting injectable bone graft substitute with examination of concomitant bone remodeling in rabbit’. European Bone and Joint Infection Society (EBJIS) 33rd Annual Meeting, Utrecht, Netherlands, September 2014.
Yang et al. ‘In vitro elution characteristics of vancomycin in a composite calcium phosphate/calcium sulfate bone substitute’. HSSJ. 2012;8:129–132.
S038/2013. Data on file, BONESUPPORT AB, Sweden.
REFERENCES for high local concentration of vancomycin, without high serum vancomycin levels
Stravinskas et al. ‘Vancomycin elution from a biphasic ceramic bone substitute.’ Bone Joint Res 2019;8:49–54.
Colding-Rasmussen et al. ‘In-vivo and in-vitro evaluation of vancomycin and gentamicin elution from bone graft substitutes’, European Bone and Joint Infection Society (EBJIS) 35th Annual Meeting, Oxford, UK, September 2016.
Soriano et al. ‘Influence of vancomycin minimum inhibitory concentration on the treatment of Methicillin-Resistant Staphylococcus aureus bacteremia’.Clin Infect Dis.2008;46:193–200.
Yap. (2019)’Vancomycin level’ http://emedicine.medscape.com/article/2090484-overview