REFERENCES for introduction text
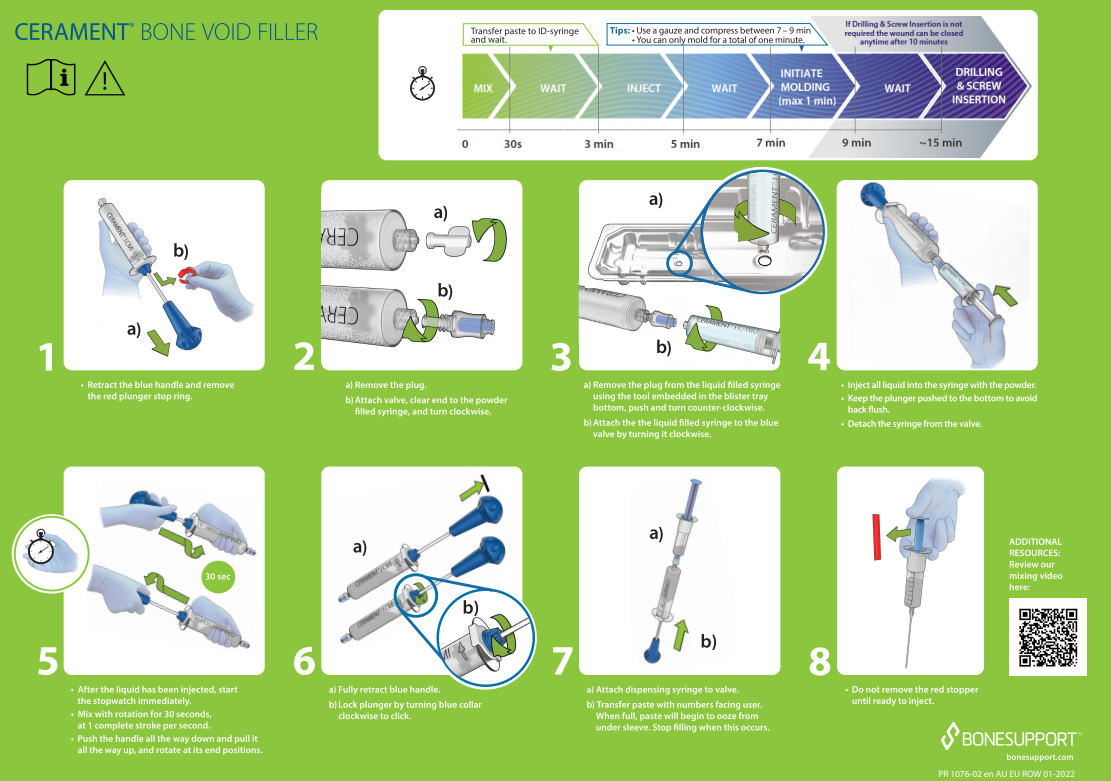
CERAMENT® BONE VOID FILLER Instructions for Use.
REFERENCES for remodels into host bone within 6-12 months
Hofmann et al. ‘Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the
Treatment of Bone Defects in Tibial Plateau Fractures – A Prospective, Randomized, Open-Label, Multicenter Study.’J Bone Joint Surg Am. 2020;102:179-93.
Hettwer et al. ‘Establishment and effects of allograft and synthetic bone graft substitute treatment of a critical size metaphyseal bone defect model in the sheep femur’. APMIS 2019; 127:53–63.
Kaczmarczyk et al. ‘Complete twelve month bone remodeling with a bi-phasic injectable bone substitute in benign bone tumors: a prospective pilot study’. BMC Musculo. Dis. 2015;16:369.
REFERENCES for injectable and flowable
CERAMENT® BONE VOID FILLER Instructions for Use.
Nilsson, M. ‘Injectable calcium sulphate and calcium phosphate bone substitutes’. Lund University. 2003. ISBN: 91-628-5603-0.
Hettwer et al. ‘Establishment and effects of allograft and synthetic bone graft substitute treatment of a critical size metaphyseal bone defect model in the sheep femur’. APMIS 2019; 127:53–63.
REFERENCES for added radiopacity
CERAMENT® BONE VOID FILLER Instructions for Use.
Nilsson et al. The composite of hydroxyapatite and calcium sulphate: a review of preclinical evaluation and clinical applications. Expert Rev. Med. Devices 2013;10(5):675-684.
REFERENCES for isothermic and not affected by room temperature
S090/2007 Data on file, BONESUPPORT AB, Sweden.
S057/2010 Data on file, BONESUPPORT AB, Sweden.
CERAMENT® BONE VOID FILLER Instructions for Use.
REFERENCES for easy to mix and use
CERAMENT® BONE VOID FILLER Instructions for Use.