Argo Knotless® Suture AnchorSuture Anchor
Argo Knotless® Suture Anchor
Ultimate Tension Control
Smart Tension Technology empowers users to adjust tension precisely, maintaining consistent suture tension from insertion to deployment. With an extended suture eyelet, Argo Knotless® anchors facilitate seamless suture loading and tensioning.
Effortless Efficiency
Experience optimized efficiency and reliability in every procedure, thanks to the cutting-edge design of Argo Knotless®. The state-of-the-art buttress thread design ensures robust fixation, minimizing the risk of anchor pullout for enhanced patient outcomes.
After anchor insertion, the Quick Release mechanism mechanically retracts the driver from the anchor body, simplifying disengagement and eliminating the need for backmalleting.
Available pre-loaded with swaged tape, Argo Knotless® shortens the repair process by reducing suture passing steps. A variety of suture color configurations provides suture differentiation, making suture management a breeze.
Want to eliminate even more steps in your procedure? Take advantage of the self-punching option and skip the step of pilot hole preparation.
Optimized Outcomes
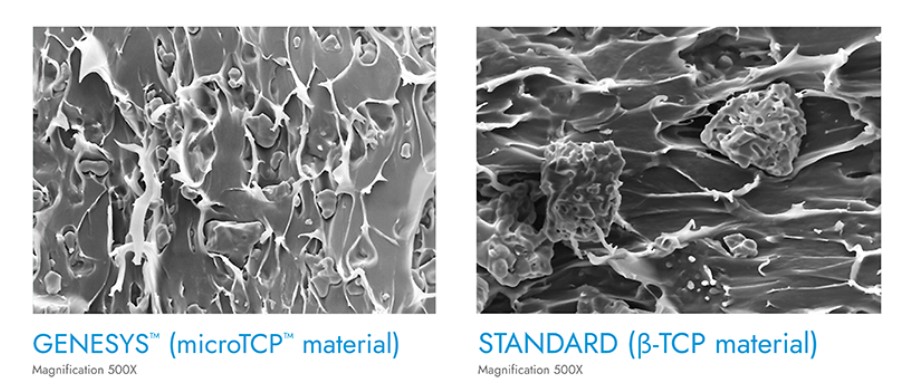
GENESYS™ Biocomposite Material
Biocomposite material combines mechanical integrity with the natural healing process, boosting material strength. The GENESYS™ material incorporates ß-TCP in it’s smallest form, known as microTCP™, which is created through a proprietary filtration process. The result is a uniformly embedded blend that boosts material strength by up to 51%.2 The GENESYS™ material provides optimized absorption rates, and may promote bone in-growth to allow for seamless integration with the body.3
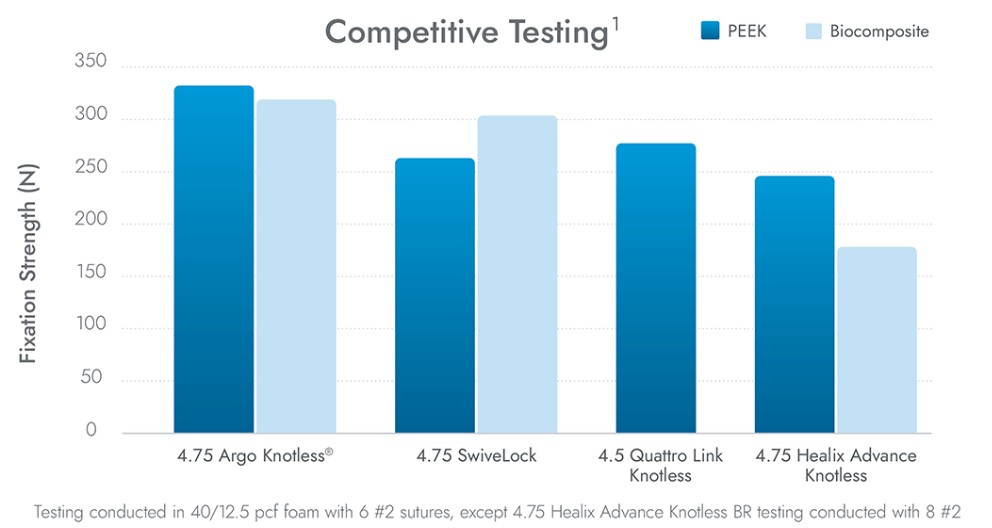
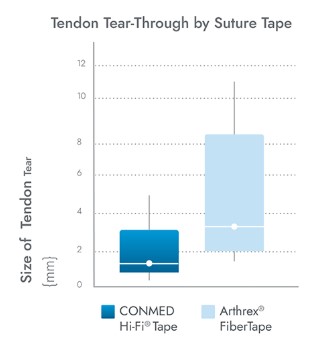
Argo Knotless® Provides Exceptional Results
CONMED’s Hi-Fi® Tape is 69% less abrasive than the leading competitor when measuring tendon tear-through, and offers broader compression than #2 suture for increased tendon-to-bone interface.4
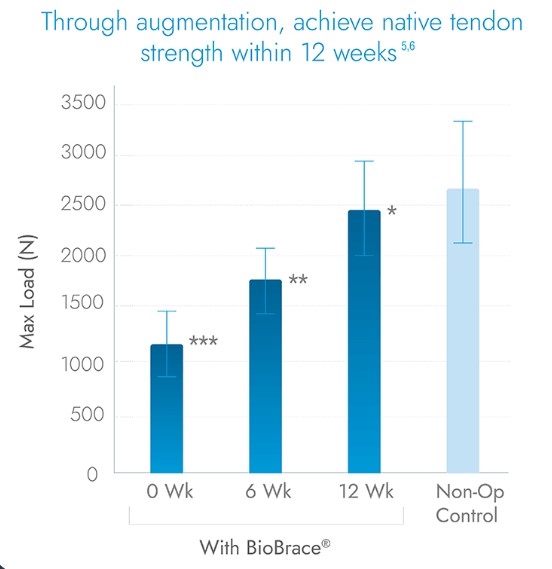
Unparalleled Strength and Healing
Step into the future of orthopedic surgery by combining Argo Knotless® GENESYS™ with CONMED’s BioBrace® technology, setting a new standard in orthopedic care. The proprietary architecture of BioBrace® features a highly porous type-1 collagen matrix reinforced with bioresorbable PLLA microfilaments.
SPECIFICATIONS
Suture Anchors
Argo Knotless® Anchor, 4.75mm, one 1.0mm Hi-Fi® Ribbon
| CAT # | K475 |
| Size | 4.75mm |
| Loaded with | 1.0mm Hi-Fi® Ribbon |
Argo Knotless® Anchor, 5.5mm, one 1.0mm Hi-Fi® Ribbon
| CAT # | K55 |
| Size | 5.5mm |
| Loaded with | 1.0mm Hi-Fi® Ribbon |
Argo Knotless® Self-Punching Anchor, 4.75mm, one 1.0mm Hi-Fi® Ribbon
| CAT # | SPK475 |
| Size | 4.75mm |
| Loaded | 1.0mm Hi-Fi® Ribbon |
Argo Knotless® Self-Punching Anchor, 5.5mm, one 1.0mm Hi-Fi® Ribbon
| CAT # | SPK55 |
| Size | 5.5mm |
| Loaded | 1.0mm Hi-Fi® Ribbon |
Drill Bit/Guide
Argo Knotless® Disposable Drill Bit, 4.75/5.5mm
| CAT # | K4755D |
| Size | 4.75/5.5mm |
Punch/Tap
Argo Knotless® Disposable Smooth Punch, 4.75/5.5mm
| CAT # | K4755DSP |
Argo Knotless® Disposable Tapered Broaching Punch, 4.75/5.5mm
| CAT # | K4755DTBP |
Argo Knotless® Disposable Tap, 4.75 mm
| CAT # | K475DTAP |
Argo Knotless® Disposable Tap, 5.5 mm
| CAT # | K55DTAP |
Argo Knotless® Reusable Smooth Punch, 4.75/5.5mm
| CAT # | K4755RSP |
Argo Knotless® Disposable Tapered Broaching Punch, 4.75/5.5mm
| CAT # | K4755RTBP |
Argo Knotless® Reusable Tap, 4.75 mm
| CAT # | K475RTAP |
Argo Knotless® Reusable Tap, 5.5 mm
| CAT # | K55RTAP |
REFERENCES
1 PDD1811996, PDD1807728.
2 TR10-496.
3 Properties of GENESYS™ Biocomposite Material Compared to Pure Polylactide (PLA). CONMED Linvatec Research and Development. 2012.
4 TR16-787. Compared with FiberTape.
5 K203267 – 510(k) Clearance Letter – The BioBrace® Implant.
6 Based on preclinical animal data.
Info Contact
| HEAD | OFFICE |
| Tel | +27 (11) 966 0600 |
| info@medhold.co.za | |
| Address | MSI Business Park, 68 Rigger Road, Spartan, Kempton Park, Gauteng, 1619 |